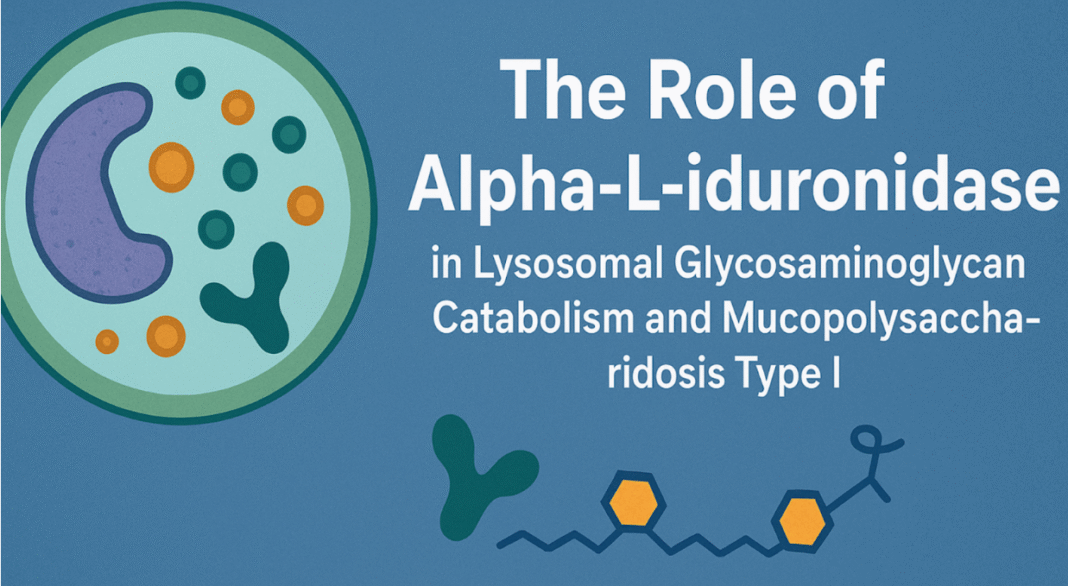
A lysosome is a small, spherical sac-like structure enclosed by a single membrane. It serves as the cell’s primary recycling centers where lysosomal catabolism takes place.
Lysosomal catabolism is the process by which complex macromolecules are broken down for reuse and excretion.
A diverse array of enzymes facilitates this intricate process. For example, Alpha-L-iduronidase (IDUA) is responsible for the ordered degradation of glycosaminoglycans (GAGs). Similarly, IDUA hydrolyzes α-L-iduronic acid residues from heparan sulfate and dermatan sulfate.
The functional IDUA enzyme has a role to play in Mucopolysaccharidosis Type I (MPS I). IDUA activity deficiency leads to accumulation of undegraded GAGs within lysosomes, which can cause widespread cellular dysfunction and tissue damage.
In this article, we will explore the fundamental biology and pathophysiology of the IDUA enzyme. We will also examine how the development of Alpha-L-iduronidase (IDUA) Recombinant Protein has advanced MPS I research.
Molecular and Cellular Biology of the IDUA Enzyme
Gene and Protein Structure
The alpha-L-iduronidase enzyme is encoded by the IDUA gene, which is located at the 4p16.3 position of the short arm of human chromosome 4. Initially, a large, inactive precursor version of the IDUA protein is made. It then becomes a working enzyme through cleavage.
The catalytically active, mature form of the enzyme can now break down other molecules. It is also a glycoprotein, as it has attached sugar molecules, which are essential for its proper folding. The protein must precisely fold into a three-dimensional conformation for stability. The folding must also create a catalytic site for chemical reactions to take place.
The Enzymatic Mechanism
The enzymatic mechanism of the IDUA enzyme involves the hydrolysis of the following two primary glycosaminoglycan (GAG) substrates:
- Dermatan sulfate
- Heparan sulfate
These two polysaccharides make the enzyme’s function highly specialized. Acting as exoglycosidase (a type of hydrolase), the enzyme removes non-reducing terminal α-L-iduronic acid residues from the GAG chains.
IDUA precisely cleaves the terminal sugar through a hydrolysis reaction, using a water molecule to break the glycosidic bond between the terminal sugar and the rest of the polysaccharide chain.
This action prepares the remaining chain for the next enzyme in the degradation process.
Post-Translational Modifications
Once the IDUA protein works as expected and gets to the lysosome, the protein is modified.
N-Glycosylation
First of all, sugar chains are attached to the IDUA protein in the endoplasmic reticulum through a process called N-glycosylation. This process helps the protein fold into its correct 3D shape, making the enzyme stable and functional.
The Mannose-6-Phosphate (M6P) Tag
After the protein is correctly folded, it is moved to the Golgi apparatus, where a specific mannose sugar on its glycan chains is tagged with a phosphate group. This creates a mannose-6-phosphate (M6P) tag, which acts like a postal code, directing the protein to the lysosome.
On its way to the lysosome, the endosome encounters a low-pH environment, where the IDUA protein is released from its receptor. The receptor is recycled back to the Golgi, and the IDUA enzyme continues on to the lysosome.
Pathophysiology of Mucopolysaccharidosis Type I (MPS I)
α-L-iduronidase (IDUA) enzyme deficiency leads to the accumulation of undegraded heparan sulfate and dermatan sulfate. This buildup triggers secondary and tertiary cellular events, driving the progressive nature of the Mucopolysaccharidosis Type I (MPS I) disease.
Lysosomal swelling and subsequent dysfunction due to the accumulating glycosaminoglycans (GAGs) disrupts cellular homeostasis. This leads to inflammation, oxidative stress, and other pathogenic cascades and also impairs cellular processes like autophagy and protein trafficking.
The Recombinant IDUA Protein: A Scientific and Therapeutic Breakthrough
The discovery of the IDUA enzyme deficiency in Mucopolysaccharidosis Type I (MPS I) has led to the development of Enzyme Replacement Therapy (ERT). This treatment provides a functional enzyme to reduce the effects of the disease.
A recombinant human alpha-L-iduronidase protein, laronidase, was the first approved ERT for MPS I.
Recombinant protein production of laronidase relies on recombinant DNA technology. The human IDUA gene is cloned into an expression vector, and the vector is transfected into a mammalian host cell line.
These cells enable the expression, folding, and post-translational modification of the enzyme.
Laronidase uses a special mannose-6-phosphate (M6P) tag to get inside cells, where it then breaks down the heparan and dermatan sulfate buildup in the lysosomes.