The desire for lighter, more even-toned skin has fueled a multibillion-dollar global skincare industry. In many cultures, fair skin has historically been equated with beauty, success, and social status. Unfortunately, this demand has given rise to a dangerous parallel market. Black market skin lightening creams, often unregulated and illegally sold, continue to circulate despite known health risks, particularly in countries across Asia, Africa, and the Middle East.
These products often contain harmful ingredients like mercury, hydroquinone, and potent corticosteroids, many of which are banned or strictly regulated. Yet, due to ease of access through street vendors, online marketplaces, and informal beauty salons, people continue to use them, sometimes unknowingly, and often at great cost to their health.
The Allure and the Risk
Skin lightening has deep social and cultural roots. For some, it represents beauty standards portrayed in media. For others, it’s a response to societal pressures or colorism. However, the pursuit of lighter skin often overlooks the hidden dangers lurking within unlabelled jars and mislabeled creams.
In 2022, the World Health Organization (WHO) estimated that skin lightening products were used by 77% of women in Nigeria, 59% in Togo, and 40% in China (WHO, 2022). A significant number of these products are purchased outside regulated channels where oversight is minimal.
What’s Inside These Creams?
Despite global bans on toxic ingredients, black market creams continue to test positive for:
-
Mercury: Used to inhibit melanin production, mercury can accumulate in the body, leading to kidney damage, neurological issues, and birth defects. It is banned in cosmetics by the Minamata Convention on Mercury (UNEP, 2013).
-
Hydroquinone: Although effective as a depigmenting agent under medical supervision, high concentrations in unregulated creams can cause exogenous ochronosis, a disfiguring, blue-black pigmentation of the skin (Olumide et al., 2008).
-
Topical corticosteroids (e.g., clobetasol propionate): These can thin the skin, cause stretch marks, acne, delayed wound healing, and systemic effects like adrenal suppression when used excessively or without prescription.
Shockingly, many users are unaware that the creams they apply nightly contain such ingredients. Labels may be in foreign languages, incomplete, or entirely absent.
Real-Life Consequences
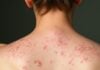
Fatima, a 27-year-old woman from Lahore, began using a “glow cream” she bought from a local market. Within weeks, her face was lighter, but also red, itchy, and swollen. After months of continued use, she developed severe steroid-induced acne and visible facial hair, a consequence of high-potency corticosteroids.
Her case is far from unique. Dermatologists worldwide are reporting increased cases of topical steroid-damaged skin (TSDS) and mercury poisoning due to cosmetic misuse. A case report in The Lancet detailed a woman who developed nephrotic syndrome after using mercury-laden cream for just two months (Chan et al., 2012).
The Problem of Regulation
Many countries have strict regulations on cosmetic ingredients, but enforcement remains a challenge. Products banned in pharmacies are readily available online or through under-the-counter sales. Border seizures only scratch the surface of a much deeper issue.
In Pakistan, for example, the Pakistan Standards and Quality Control Authority (PSQCA) has banned fairness creams with more than 1 ppm of mercury. Yet, tests by the Sustainable Development Policy Institute (SDPI) found that over 56% of creams in local markets exceeded this limit, with some containing over 20,000 times the permissible amount (SDPI, 2018).
The situation is made worse by celebrity endorsements and social media influencers who promote fairness products without disclosing their contents or side effects.
A Call for Awareness and Safe Alternatives
Combatting the problem requires a multi-faceted approach:
-
Education: Raising awareness is crucial. Many consumers don’t know the risks or how to read ingredient labels. Campaigns like the WHO’s “Stop Using Mercury-Added Products” aim to inform users, particularly women of childbearing age.
-
Stronger enforcement: Border control, stricter penalties for importers, and crackdowns on illegal vendors are essential steps.
-
Support from the beauty industry: Brands must promote inclusive beauty and move away from terms like “fairness” and “whitening,” which reinforce harmful beauty standards.
-
Dermatologist-guided care: For individuals suffering from pigmentation disorders like melasma or post-inflammatory hyperpigmentation, safe and effective treatments like azelaic acid, kojic acid, and vitamin C serums are available under medical guidance.
The continued circulation of black market skin lightening creams is a public health issue hiding in plain sight. Beyond cosmetic harm, these products pose real risks to kidney, endocrine, and neurological health. Addressing this growing problem requires a collaborative effort between regulatory agencies, healthcare professionals, and the beauty industry, alongside empowering individuals to embrace diverse definitions of beauty.
References
- Chan, T. Y. K., & Chan, A. Y. W. (2012). Nephrotic syndrome and skin lightening cosmetics containing mercury. The Lancet, 379(9817), 684. https://doi.org/10.1016/S0140-6736(12)60274-5
- Olumide, Y. M., Akinkugbe, A. O., Altraide, D., Mohammed, T., Ahamefule, N., Ayanlowo, S., Onyekonwu, C., & Essen, N. (2008). Complications of chronic use of skin lightening cosmetics. International Journal of Dermatology, 47(4), 344–353. https://doi.org/10.1111/j.1365-4632.2008.02509.x
- Sustainable Development Policy Institute (SDPI). (2018). Mercury levels in fairness creams in Pakistan. Retrieved from https://sdpi.org
- United Nations Environment Programme (UNEP). (2013). Minamata Convention on Mercury. Retrieved from https://www.mercuryconvention.org/
- World Health Organization (WHO). (2022). Skin lightening products: Facts, risks, and awareness. Retrieved from https://www.who.int