Stevens-Johnson Syndrome (SJS) is a rare but serious disorder that affects the skin and mucous membranes. It is considered a medical emergency because it can progress rapidly and cause life-threatening complications. In recent years, researchers have made significant progress in understanding the pathophysiology or the biological processes behind SJS. These advances are helping doctors better identify, treat, and potentially prevent the condition.
Understanding SJS: A Quick Overview
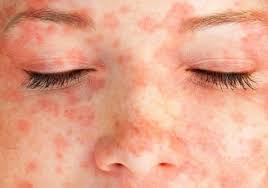
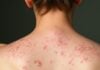
SJS is usually triggered by medications such as antibiotics (especially sulfonamides), anti-seizure drugs, and nonsteroidal anti-inflammatory drugs (NSAIDs). It starts with flu-like symptoms, followed by a painful red or purplish rash that spreads and blisters. The top layer of skin may peel away, similar to a severe burn. In severe cases, SJS progresses to toxic epidermal necrolysis (TEN), a more extreme form involving more than 30% of the body surface area (Schwartz et al., 2013).
Genetic Susceptibility: The Role of HLA Alleles
One of the most important advances in the pathophysiology of SJS is the discovery of genetic predispositions. Certain genes, especially those in the human leukocyte antigen (HLA) system, increase a person’s risk of developing SJS in response to specific drugs. For instance, the HLA-B*15:02 gene is strongly associated with carbamazepine-induced SJS, especially in individuals of Southeast Asian descent (Chung et al., 2004). Similarly, HLA-B*58:01 has been linked to allopurinol-induced SJS (Hung et al., 2005).
These discoveries have led to the development of screening tools that help doctors avoid prescribing high-risk drugs to genetically susceptible individuals. In countries like Thailand and Taiwan, HLA screening before starting certain medications has already lowered the incidence of drug-induced SJS significantly.
Immune System Misfire: The Cytotoxic T-Cell Response
Recent studies have confirmed that SJS is not just a skin problem it’s a type of immune reaction. More specifically, it’s classified as a delayed-type hypersensitivity reaction. When a susceptible individual takes a certain drug, their immune system mistakenly identifies the drug or its metabolites as a threat. Cytotoxic T-cells, which usually attack virus-infected cells, become overly activated and start targeting healthy skin and mucosal cells (Mockenhaupt, 2014).
These T-cells release enzymes like granulysin, perforin, and granzyme B. Granulysin has been shown to play a central role in killing keratinocytes (skin cells), leading to the widespread skin damage seen in SJS and TEN (Chung et al., 2008). Elevated granulysin levels have even been proposed as a potential biomarker for early detection.
Drug Metabolism and Reactive Metabolites
Another key area of progress involves the way certain drugs are metabolized in the body. Some individuals have specific variations in liver enzymes that lead to the accumulation of reactive drug metabolites. These unstable compounds can bind to proteins and trigger an immune response, contributing to the onset of SJS (Pirmohamed, 2011).
Additionally, the formation of these metabolites may depend on environmental and personal factors such as infections, co-medications, and overall immune status. Understanding these pathways is helping scientists identify which drugs are more likely to cause SJS and in whom.
The Skin Barrier and Keratinocyte Apoptosis
At the cellular level, researchers have learned more about how skin cells (keratinocytes) die in SJS. Apoptosis or programmed cell death is a natural process, but in SJS, it is abnormally increased. This is largely due to immune mediators like tumor necrosis factor-alpha (TNF-α) and Fas ligand. These proteins send “death signals” to keratinocytes, causing them to self-destruct (Viard et al., 1998).
The integrity of the skin barrier is lost, making patients vulnerable to infection and fluid loss. Current research is focusing on finding drugs that can block these apoptotic pathways, potentially limiting the severity of SJS.
Microbiome and Infections
Emerging evidence suggests that the microbiome the collection of microbes living on and in our bodies may also play a role. Some infections, especially with Mycoplasma pneumoniae, are known to trigger SJS in children. Researchers are now exploring how shifts in the microbiome could influence immune responses and susceptibility (Pichler & Tilch, 2004). While this area is still developing, it may open up new preventive strategies in the future.
Thanks to recent advances in genetics, immunology, and molecular biology, our understanding of the pathophysiology of Stevens-Johnson Syndrome has grown tremendously. From identifying high-risk genes like HLA-B*15:02 to recognizing the central role of cytotoxic T-cells and granulysin, science is paving the way for more targeted prevention and treatment strategies. With ongoing research, there’s hope that SJS can eventually be predicted and even prevented before it ever begins.
References
- Chung, W. H., Hung, S. I., Hong, H. S., Hsih, M. S., Yang, L. C., Ho, H. C., … & Chen, Y. T. (2004). Medical genetics: a marker for Stevens–Johnson syndrome. Nature, 428(6982), 486.
- Chung, W. H., Wang, C. W., Dao, R. L. (2008). Severe cutaneous adverse drug reactions. The Journal of Dermatology, 35(12), 735-746.
- Hung, S. I., Chung, W. H., Liou, L. B., Chu, C. C., Lin, M., Huang, H. P., … & Chen, Y. T. (2005). HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proceedings of the National Academy of Sciences, 102(11), 4134–4139.
- Mockenhaupt, M. (2014). The current understanding of Stevens–Johnson syndrome and toxic epidermal necrolysis. Expert Review of Clinical Immunology, 7(6), 803-813.
- Pichler, W. J., & Tilch, J. (2004). The role of infections in the pathogenesis of adverse drug reactions. Trends in Pharmacological Sciences, 25(4), 178-183.
- Pirmohamed, M. (2011). Drug-induced hypersensitivity reactions: understanding the pathogenesis. British Journal of Clinical Pharmacology, 71(5), 682–692.
- Schwartz, R. A., McDonough, P. H., & Lee, B. W. (2013). Toxic epidermal necrolysis: Part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. Journal of the American Academy of Dermatology, 69(2), 173.e1-173.e13.
- Viard, I., Wehrli, P., Bullani, R., Schneider, P., Holler, N., Salomon, D., … & Tschopp, J. (1998). Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science, 282(5388), 490-493.